The U.S. Food & Drug Administration (FDA) announced that it is expanding its recommendation and recalling all ByHeart infant formula products due to the number of infants becoming ill.
All ByHeart Whole Nutrition Infant Formula products have been recalled, including their cans and single-serve sticks.
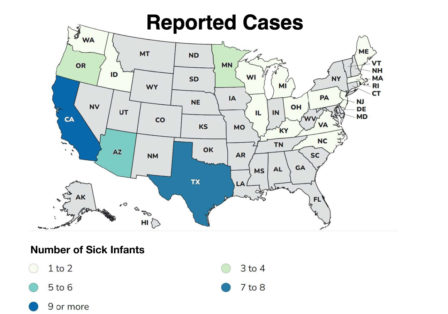
As of December 11, 2025, 51 infants across 19 states have become ill with infant botulism linked to the product. Note, though, that the FDA has not identified a direct link between ByHeart or any other infant formulas causing infant botulism.
Symptoms of Infant Botulism
Most infants with infant botulism will develop constipation, poor feeding, loss of head control, and difficulty swallowing. This can progress to difficulty breathing. Symptoms can take several weeks to develop.
What To Do If You Have The Recalled Baby Formula
Parents and caregivers should stop using any ByHeart infant formula products immediately. If your child is experiencing any symptoms, immediately contact your care provider. If you have questions regarding this recall, contact ByHeart at hello@byheart.com. For more information on the recall, you can also visit byheart.com or call 1-833-429-4327
If you have the formula in your house, take a photo or record the information on the bottom of the package. Keep it in a safe spot labeled Do Not Use. If your child develops symptoms, contact your state health department with the product information. If your child does not develop symptoms after 30 days, throw out the product containers.
Affected Product Photos – Updated


Recall Map
Reported Cases – Updated